Is Agno3 Nacl Agcl Nano3 an Oxidation Reduction Reaction
Redox reactions involve formal electron transfer and a FORMAL change in oxidation number. Ag 1 N 5 O -2.

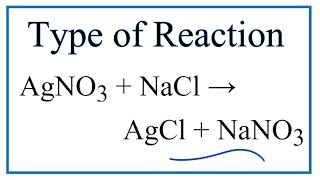
Type Of Reaction For Agno3 Nacl Agcl Nano3 Youtube
We can identify the oxidation and reduction half-reactions.

. In the reaction agno3 aq nacl aq nano3 aq agcl s the reactants a. 1 A redox reaction is an oxidation-reduction reaction. What will you observe when NaCl is treated with AgNO3.
Because calculated value for Ksp expression is greater than Ksp value of AgCl. OKOH HNO3 H2O KNO3 ON2 O2 2NO AgNO3 NaCl AgCl NaNO3 CaCl2 Na2SO4 CaSO4 2NaCl Al2SO43 6KOH 2AlOH3 3K2504 Question 3 Calculate the molar mass of magnesium chloride MgCl 2. C N2 O2 ---- 2NO.
NaClAgNO3NaNO3AgCl is not a oxidation-reduction reaction because there is no change in oxidation state of any element. Iv In FeCl₂g the oxidation state of Cl is 1- and the oxidation state of Fe is 2-. AgCl is a precipitate and does not cause for hydrolysis reaction.
Ag 1 Cl -1. No Because nothing has been oxidised or reduced. Al2SO43 6KOH 2AlOH3 3K2SO4 e.
Estimate the temperature where Delta G 0 for the following reaction Given. Ksp AgCl 17 10-10 mol 2 dm-6. Both NaCl and AgNO 3 are neutral solutions.
Oxidation numbers of the individual element on the reactant side. A AgNO3 NaCl AgCl NaNO3 B Cl2 H2O HClO HCl C CuO CO CO2 Cu D NaOH HCl NaCl H2O Which two reactions are redox. Oxidation involves the LOSS of electrons OIL.
When silver nitrate solution is added to sodium chloride solution then a white precipitate of silver chloride is formed alongwith sodium nitrate solution. A CaCl2 Na2SO4 ----- CaSO4 2NaCl. N2 O2 2NO.
NaCl AgNO3 AgCl NaNO3 NaNO2 HCI - NaCl HNO2 CaSO3 2HCl - CaCl2 H20S02 3CuO 2NH3 - 3Cu 3H2O N2 Use Faradays constant to calculate the charge in Coulombs used during the electrolysis for 000964 mol e F 96500 coulombs per mole. In a double displacement reaction the anions are exchanged and in a precipitation reaction a precipitate is formed in the end. The products of the reaction are AgCl and NaNO 3.
In this case AgCl is the precipitate. Oxidation numbers of the individual element on the product side. Which one of the following reactions is an oxidation reduction reaction.
The reaction AgNO3aq NaClaq AgCls NaNO3aq is an reaction. Which of the following is not oxidation reaction. KOH HNO3 H2O KNO3 b.
Which of the following is an oxidation-reduction reaction. 2 H₂Ol NOg NO₃aq 4Haq 3 e The standard cell potential E is equal to the difference between the standard reduction potential of the cathode and the standard reduction potential of the anode. In a double displacement reaction the anions are exchanged and in a precipitation reaction a precipitate is formed in the end.
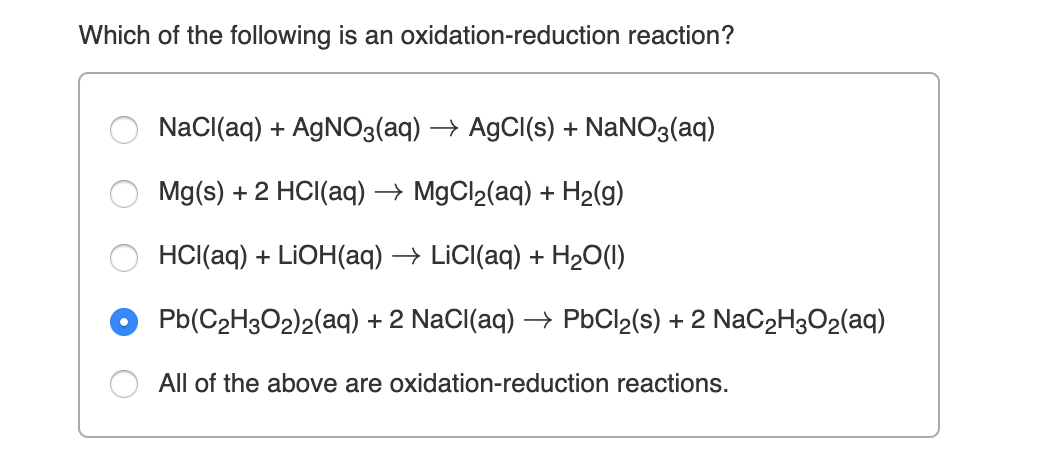
1 e AgCls Ags Claq Oxidation anode. In this set of chemical reactions which reaction is an oxidation-reduction reaction. A K2SO4 BaCl2 BaSO4 2KCl b CuSO4 BaCl2 BaSO4 CuCl2 c Zn H2SO4 ZnSO4 H2 d PbNO32 2NaCl PbCl2 2NaNO3 e AgNO3 NaCl AgCl NaNO3.
AgNO3aq NaClaq AgCls NaNO3aq A 1079 g B 1699 g C 844 g D 0589 g E 589 g. So we write the individual reactions with the oxidation number of each constituent species superscripted. Prafull added an answer on 18713.
2 An oxidation-reduction reaction is one in which at least one species is oxidized and other or others is reduced. Which of the following is an oxidation-reduction reaction. AgNO3 NaCl AgCl NaNO3 c.
NaCl AgNO 3 AgCl NaNO 3. 4 Reduction is the decrease of the oxidation number which occurs by gaining. Grades 10 12 Redox Balancing Redox Reactions Practice Worksheet In This Hands On Worksheet Students Will Be R Redox Reactions Reactions Practices Worksheets The loss of electrons results in an increase in charge or oxidation state.
The equation for the reaction is below. When solutions of AgNO3 and NaCl react the balanced molecular equation is. There is no change in the oxidation numbers hence it is not an oxidation-reduction reaction.
AgNO3 NaCl AgCl NaNO3. Consider the equations A B C and D. AgNO3aq NaClaq AgCls NaNO3aq How much AgCl is produced when 310 g of AgNO3 and 0600 g of NaCl react.
Correct options are A and B This reaction is both precipitation reaction as well as double displacement reaction. AgNO3aq NaClaq AgCls NaNO3a. In this case AgCl is.
During this reaction the cations and anions of two different compounds switch places forming two entirely different compounds. In this video we determine the type of chemical reaction for the equation AgNO3 NaCl AgCl NaNO3 Silver Nitrate and Sodium Chloride. Therefore you can see AgCl is precipitated in the solution.
Na 1 Cl -1. PH of NaCl and AgNO 3 reaction. AgNO₃aq NaClaq AgCls NaNO₃ Compound spieces oxidation state.
G of silver nitrate when it is mixed with an excess of sodium chloride. Double displacement reactions may be defined as the chemical reactions in which one component each of both the reacting molecules is exchanged to form the products. Both oxidation and reduction d.
Double displacement reaction is not an oxidation reaction. Delta H-176 and Delta S -2845 JK NH3 HCl -- NH4Cl. StackrelIHstackrel-IClaqstackrelINa stackrel-IIOstackrelIHaq rarr stackrelINa stackrel-I Claq.
None of the elements changed its oxidation state which means that this is not an oxidation-reduction reaction. Certified by MeritNation Expert. 3 Oxidation is the increase on the oxidation number which occurs by releasing electrons.
CaCl2 Na2SO4 CaSO4 2NaCl d. Asked Jun 30 2017 in Environmental Atmospheric Sciences by Marcia. Solve any question of Chemical Reactions and Equations with-.
Since we have two. Which of the following is an oxidation reduction reaction. This reaction is both precipitation reaction as well as double displacement reaction.
None of these c. B KOH HNO3 ---- H2O KNO3. In the reaction of silver nitrate with sodium chloride how many grams of silver chloride will be produced from 100.
Sodium chloride NaCl reacts with silver nitrate AgNO3 to produce silver chloride AgCl and sodium nitrate NaNO3. Is a redox reaction.

There Are 4 Main Types Of Chemical Reactions Synthesis Decomposition Single Displacement And Double Disp Chemistry Lessons Chemical Reactions Reaction Types
Type Of Reaction For Agno3 Nacl Agcl Nano3 Youtube
Solved Which Of The Following Is An Oxidation Reduction Chegg Com
Comments
Post a Comment